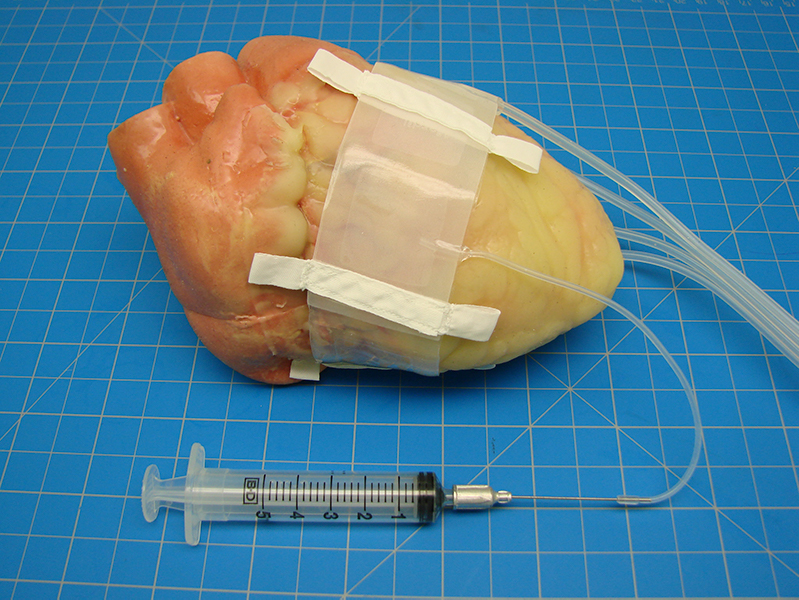
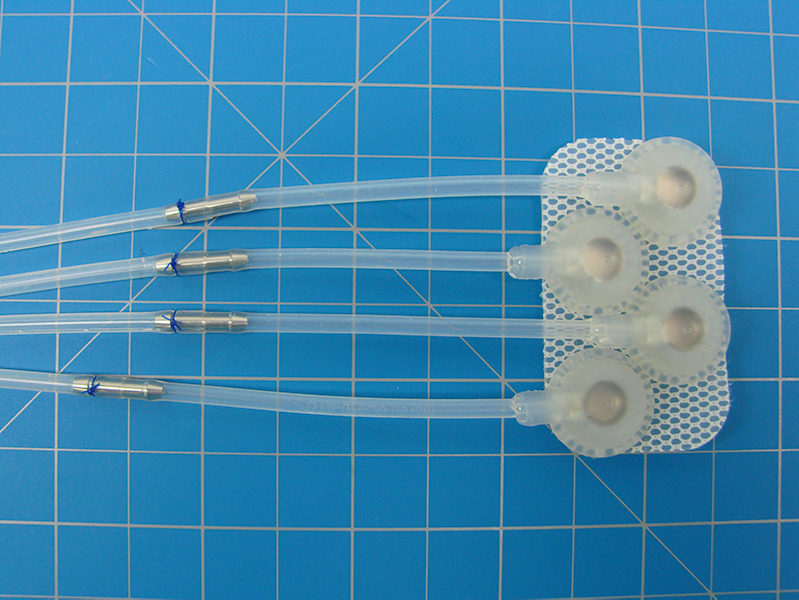
ATech Designs, LLC and Phoenix Cardiac Devices, Inc. receives CE mark and approval to sell their mitral valve device in Europe effective April 1, 2021 to May 27, 2024. The BACE device was developed to provide a more effective and less invasive method of treating mitral regurgitation that reduces complications and thus lowers the risk of mortality.
Phoenix Cardiac (ATech Designs, LLC’s client of 14 years) is likely the first company and only company in the world to achieve a CE mark for a Class III medical device as a virtual company. Founder of ATech Designs, LLC Karl R. Leinsing, MSME, PE, acted as Vice President of Engineering for Phoenix. ATech Designs, LLC was responsible for all the engineering work on the device including testing, validation, verification, and some limited manufacturing. It was a team effort from many vendors outside of ATech Designs, LLC and the team at ATech Designs, LLC including Amy McMath, Joseph Durant, and Thomas Lefebvre.
This is a great achievement for our team!
More information on the device can be found at www.phoenixcardiac.com.
Related Links:
https://citoday.com/news/phoenix-cardiac-devices-begins-study-of-bace-mitral-device
https://www.biospectrumasia.com/news/27/354/phoenixs-heart-repair-device-trial-is-underway.html